FDA puts new limits on Johnson & Johnson vaccine due to rare blood clotting condition
Health officials have detected 60 cases of the rare blood clotting condition TTS, nine of which have been fatal
Blood clotting from Johnson & Johnson vaccine 'extremely rare': Dr. Nesheiwat
Dr. Janette Nesheiwat on Johnson & Johnson vaccine
The FDA revised its emergency authorization to sharply limit the use of Johnson & Johnson's COVID-19 vaccine due to a rare but potentially fatal blood clotting condition called thrombosis with thrombocytopenia syndrome (TTS).
Only individuals who are 18 years of age and older who "would otherwise not receive a COVID-19 vaccine" due to availability or their choice should now take Johnson & Johnson's one-shot regiment.
"Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals," Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, said in a statement Thursday.

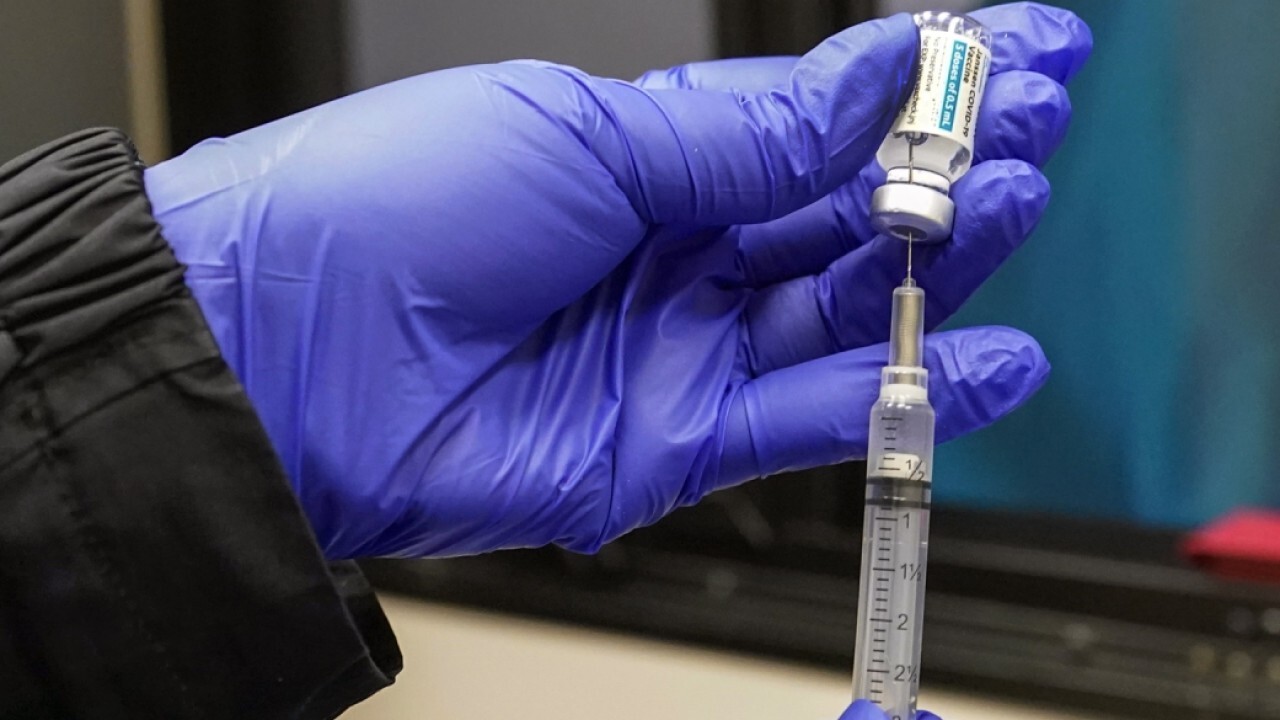
Syringes filled with the Johnson & Johnson vaccine are shown, Thursday, May 13, 2021, at a mobile vaccination site at the Greater Bethel Church in Miami. (AP Photo/Wilfredo Lee / AP Newsroom)
"We’ve been closely monitoring the Janssen COVID-19 Vaccine and occurrence of TTS following its administration and have used updated information from our safety surveillance systems to revise the EUA," Marks added.
COVID TESTS, VACCINES HELP WALGREENS BEAT EXPECTATIONS ON EARNINGS
The CDC's Advisory Committee on Immunization Practices unanimously voted in December to give a preferential recommendation to Pfizer and Moderna's two-shot mRNA vaccines.
TTS is a syndrome of blood clotting in combination with low levels of platelets, which are the blood cells that help your body halt bleeding.

In this file photo, large banners hang in an atrium at the headquarters of Johnson & Johnson in New Brunswick, N.J. (AP Photo/Mel Evans, File / AP Images)
Health officials have confirmed 60 cases of TTS following Johnson & Johnson's vaccine, nine of which have been fatal.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Women between the ages of 30 and 49 are most vulnerable to the blood clotting disorder, according to the CDC.
About 18.7 million doses of Johnson & Johnson's vaccine have been administered in the United States.