FDA authorizes Pfizer booster shots for kids ages 5-11
Booster shot would be administered at least 5 months after completion of a primary series with Pfizer-BioNTech vaccine
The U.S. Food and Drug Administration (FDA) announced Tuesday that it had amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine, allowing the use of a single booster dose in children ages 5 to 11.
FDA PUTS NEW LIMITS ON JOHNSON & JOHNSON VACCINE DUE TO RARE BLOOD CLOTTING CONDITION
The shot would be given at least five months after completion of a primary series with the Pfizer-BioNTech vaccine.
"The FDA is authorizing the use of a single booster dose of the Pfizer-BioNTech COVID-19 vaccine for children 5 through 11 years of age to provide continued protection against COVID-19," FDA Commissioner Dr. Robert Califf said in a statement."
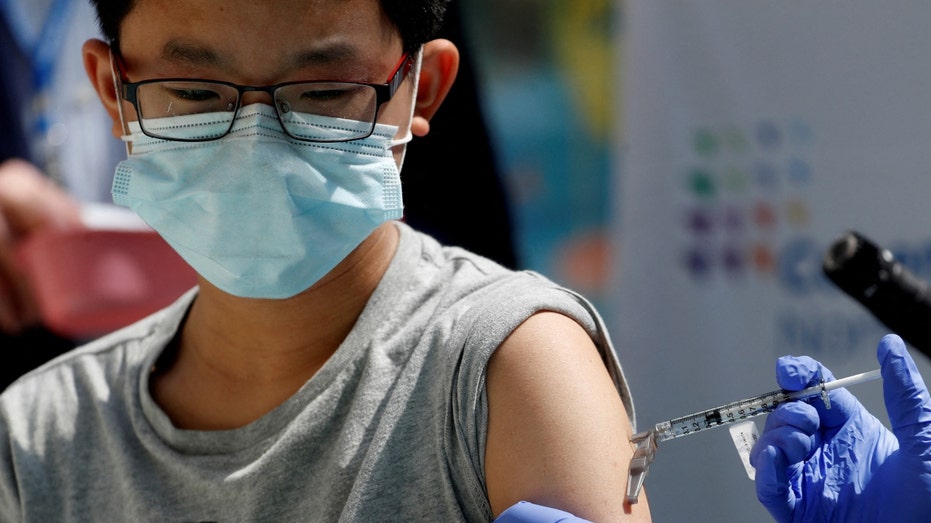
Brendan Lo (13) receives a dose of the Pfizer-BioNTech vaccine for the coronavirus disease (COVID-19) at Northwell Health's Cohen Children's Medical Center in New Hyde Park, New York, U.S., May 13, 2021. (REUTERS/Shannon Stapleton / Reuters)
"While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer term effects, even following initially mild disease," Califf said.
PFIZER TO BUY REST OF BIOHAVEN FOR $11.6B
To make its decision, the FDA said it analyzed immune response data in a subset of children from an ongoing randomized placebo-controlled trial that supported the Oct. 2021 authorization of the Pfizer-BioNTech primary series in the same age group.
"Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 vaccine. The antibody level against the SARS-CoV-2 virus one month after the booster dose was increased compared to before the booster dose," it said in a release.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The safety of a single booster dose of the vaccine in children ages 5-11 was assessed in approximately 400 children, with side effects including redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills and fever.
FDA limits J&J COVID-19 vaccine for adults
Fox News contributor Dr. Marty Makary weighs in on the FDA limiting the use of J&J COVID-19 vaccines for adults.
"The FDA has determined that the known and potential benefits of a single booster dose of the Pfizer-BioNTech COVID-19 vaccine for children 5 through 11 years of age at least five months after completing a primary series outweigh its known and potential risks and that a booster dose can help provide continued protection against COVID-19 in this and older age groups," Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said.
In January, the FDA authorized the use of a single booster dose of the Pfizer-BioNTech vaccine for administration to kids 12-15 years of age after completion of primary vaccination with the Pfizer-BioNTech vaccine.
CLICK HERE TO READ MORE ON FOX BUSINESS
The FDA has authorized the Pfizer-BioNTech vaccine for use in individuals 5 years of age and older.
Pfizer announced in April that it would ask the FDA to authorize the booster dose for emergency use in the group.
Competitor Moderna asked FDA regulators to authorize its vaccine for emergency use in children under 6.






















