FDA greenlights Abbott's new $5 coronavirus test that provides results within 15 minutes
Test is the size of a credit card, administered by healthcare professionals using a nasal swab.
XpresSpa partners with Abbott Labs to offer 15-minute coronavirus tests at airports
XpresSpa Group CEO Doug Satzman discusses providing travelers and employees at airports with 15-minute coronavirus tests and how his company is doing financially.
Abbott Laboratories has been given the greenlight by the Food and Drug Administration for an emergency use authorization for its $5 rapid antigen test for the coronavirus.
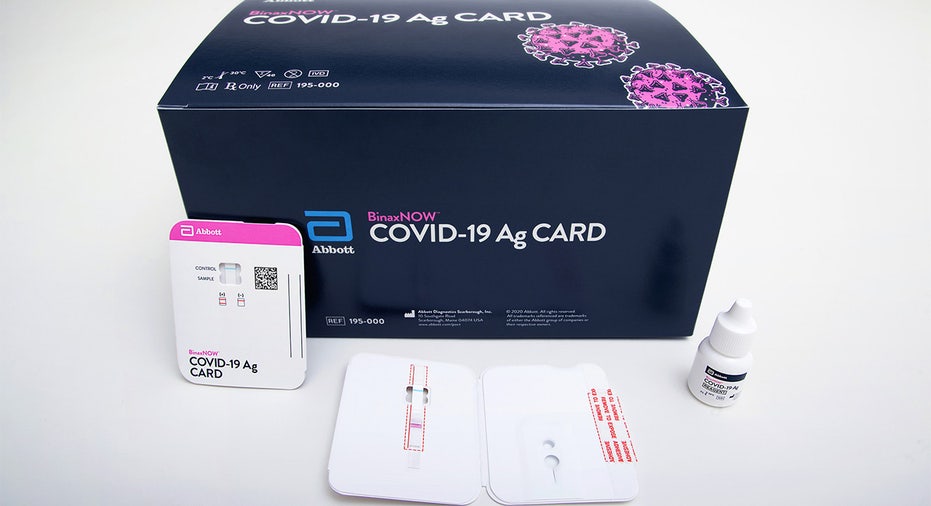
Abbott's BinaxNOW™ COVID-19 Ag Card is a rapid, reliable and affordable tool for detecting active coronavirus infections at massive scale. (Photo courtesy of Abbott Laboratories)
The test, called the BinaxNOW COVID-19 Ag Card, is roughly the size of a credit card and is administered by a healthcare professional using a nasal swab. BinaxNow uses lateral flow technology, similar to an at-home pregnancy test, to search for virus proteins and is intended to be used for patients within seven days of feeling coronavirus symptoms. The test can provide results in up to 15 minutes.
According to Abbott, the test has a demonstrated sensitivty, or the percent of positive cases that it can accurately detect, of about 97%.
GOOGLE CLOUD INVESTING $100M INTO TELEHEALTH PLATFORM AMWELL
In addition to increasing overall testing capabilities and result turnaround times, BinaxNow is aimed at helping bring employees back to work and children back to school.
BinaxNow can be linked to a free app called NAVICA, which allows those who are given the "all clear" to display a digital health pass via a QR code that displays their negative test result.

NAVICA™ is a no-charge complementary phone app, which allows people to display their BinaxNOW test results. (Photo courtesy of Abbott Laboratories)
"We intentionally designed the BinaxNOW test and NAVICA app so we could offer a comprehensive testing solution to help Americans feel more confident about their health and lives," Abbott president and chief executive officer, Robert B. Ford, said in a press release Wednesday evening.
The combination of the BinaxNOW test and the NAVICA app Ford added should help provide "a bit more normalcy in our daily lives."
The FDA's emergency use authorization will allow doctors, nurses, school nurses, medical assistants and technicians, pharmacists and employer occupational health specialists to administer the test with minimal training and a patient prescription.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Abbott will ship tens of millions of tests starting in September, with plans to ramp up to 50 million tests a month at the beginning of October.
BinaxNOW is the sixth test that Abbott has launched in the U.S. to help fight the coronavirus pandemic. To date, the company has provided more than 27 million COVID-19 tests in the United States, including 14 million detection tests and 13 million antibody tests.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| ABT | ABBOTT LABORATORIES | 111.07 | +0.24 | +0.22% |
Shares of Abbott surged more than 11% in after-hours trading Wednesday evening on the news.