Novo Nordisk takes legal action against pharmacies selling drugs claiming to contain semgaglutide
The FDA has received adverse event reports and complaints after patients used compounded products claiming to contain semaglutide, Novo Nordisk said
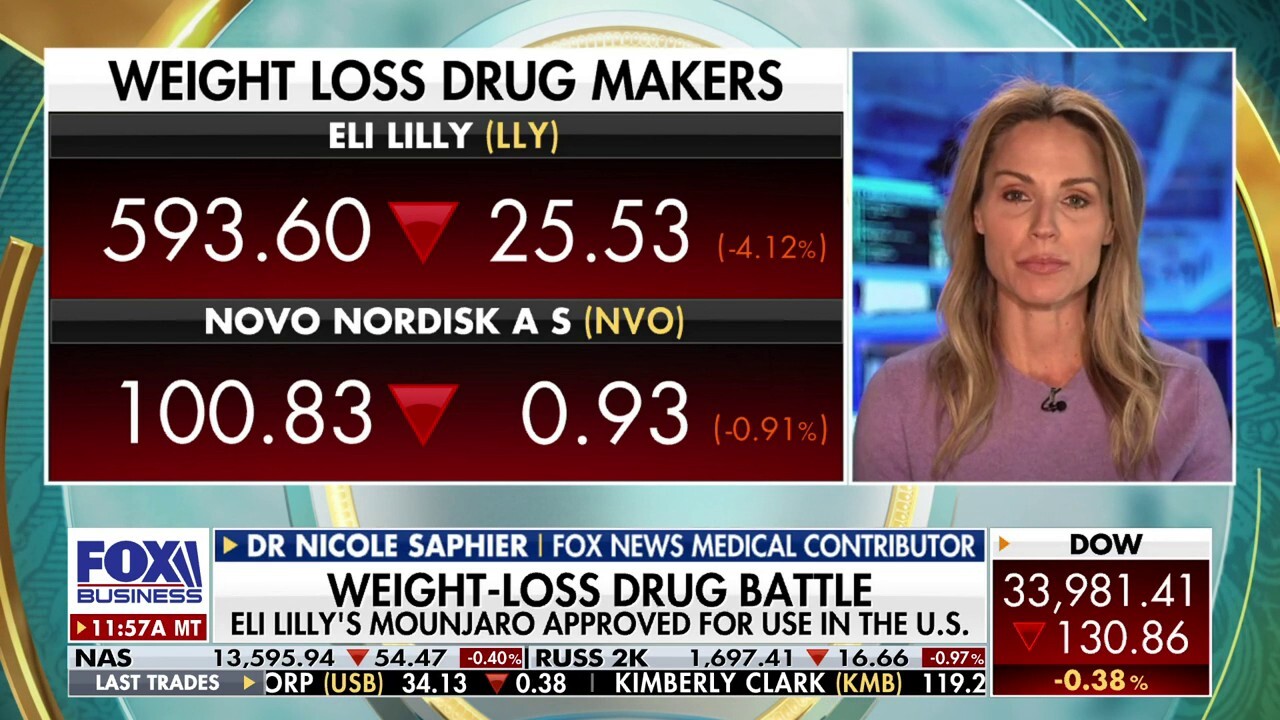
Weight-loss drugs like Ozempic are being overutilized: Dr. Nicole Saphier
FOX News medical contributor Dr. Nicole Saphier joins 'The Big Money Show' to discuss whether weight loss drugs like Ozempic and Mounjaro are safe.
Novo Nordisk – the Danish drugmaker behind Wegovy and Ozempic – is taking legal action against certain pharmacies for allegedly selling drugs claiming to contain semgaglutide that aren't approved by the U.S. Food and Drug Administration (FDA).
The compounded drugs, Novo Nordisk warned, could pose risks to patients.
On Thursday, the company accused compounding pharmacies Wells Pharmacy and Brooksville Pharmaceuticals for selling "adulterated and misbranded injectable compounded drugs that claim to contain semaglutide" which is the same active ingredient in both Wegovy and Ozempic.
Wegovy and Ozempic are among the drugs that rose to popularity on social media amid reports that celebrities were using them to shed excess weight.
DIABETES DRUG TESTED FOR WEIGHT LOSS COULD BE GAME CHANGER

The headquarters of Danish pharmaceutical company Novo Nordisk in Copenhagen, Denmark. (REUTERS/Tom Little/File photo / Reuters Photos)
Jason Brett, executive director of Medical Affairs at Novo Nordisk Inc. said that several tests of the compounded drugs that claimed to contain semaglutide showed "concerning levels of unknown impurities." One sample in particular had a concerning level of 33%, which may pose potential safety risks to patients, according to Novo Nordisk.
NOVO NORDISK'S WEGOVY REDUCES RISK OF SERIOUS HEART PROBLEMS BY 20%, STUDY SHOWS
Gastroenterologist and obesity medicine specialist Dr. Christopher McGowan told FOX Business that there is a "flourishing industry of unregulated, compounded versions of semaglutide that patients may not understand are vastly different than the brand-name, FDA-regulated drugs."
Novo Nordisk said on Thursday that it is seeking to secure court orders to prevent these two compounding pharmacies from continuing their unlawful sales of adulterated and misbranded drugs claiming to contain semaglutide.
This photograph taken on February 23, 2023, in Paris, shows the anti-diabetic medication "Ozempic" (semaglutide) made by Danish pharmaceutical company Novo Nordisk. (JOEL SAGET/AFP via Getty Images / Getty Images)
It is also trying to stop Wells Pharmacy from allegedly using false and misleading statements" such as "claiming or implying that its compounded drug claiming to contain semaglutide has been approved by FDA" as well as that "its product has been subjected to the same clinical studies and trials as Novo Nordisk’s FDA-approved semaglutide medicines."
Brooksville Pharmaceuticals disputes the allegations made and said that its attorneys are filing another motion to dismiss.
"We purchase all of our bulk ingredients, including semaglutide, from FDA-registered facilities," the company told FOX Business in a statement. "We have also tested our semaglutide formulation with a third party lab and found it to remain potent for 180 days when stored in a refrigerator, 90 days when stored at room temperature and 45 days when stored at 40 degrees Celsius."
The company further argued that state-licensed compounding pharmacies play a "critical role in providing thousands of patients across the United States with an essential medication."
Representatives for Wells Pharmacy has not immediately responded to FOX Business request for comment.
However, Novo Nordisk said that the FDA has already received an increasing number of adverse event reports and complaints after patients used compounded products claiming to contain semaglutide.
Semaglutide is part of a class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists, according to the FDA. It prompts the body to produce more insulin, reducing blood glucose. At higher levels, it can help to reduce appetite and signal a feeling of fullness, the FDA said.
Packets of Wegovy move along a conveyor at the Novo Nordisk A/S production facilities in Hillerod, Denmark, on Monday, June 12, 2023. (Carsten Snejbjerg/Bloomberg via Getty Images / Getty Images)
Its ability to help patients lose weight sparked caused a rush of demand and, subsequently, supply issues. The shortage caused people to lean on drugs like Ozempic off-label for weight loss.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
However, Novo Nordisk has repeatedly warned that neither Ozempic or Wegovy is intended to be a lifestyle medication.
The company has also said that it has taken steps to ensure the responsible use of its semaglutide medicines, which also includes the oral medication, Rybelsus, and that patients have a safe and positive experience with its FDA-approved semaglutide medicines."