FDA to meet over Moderna, Johnson & Johnson vaccine booster shots
A decision on the matter is not expected for at least another week
The U.S. Food and Drug Administration (FDA) will meet this week to debate whether or not extra doses of the Moderna, Inc. and Johnson & Johnson Janssen COVID-19 vaccines should be distributed, to whom and when.
The agency's advisers will give their recommendation on the matter before the FDA makes an official decision on booster shot authorization – which has already been the subject of some disagreement.
FULLY VACCINATED AND HAD COVID-19? NO RUSH FOR A BOOSTER SHOT, EXPERTS SAY
The answer is not expected for at least another week and next week a U.S. Centers for Disease Control and Prevention (CDC) panel will meet to go over details, with its decision subject to approval by CDC Dir. Rochelle Walensky.
In September, a CDC advisory panel OKed boosters of Pfizer's vaccine at the six-month point for individuals 65 years and older, individuals ages 18 to 64 who are at "high risk of severe COVID-19" and nursing home residents.
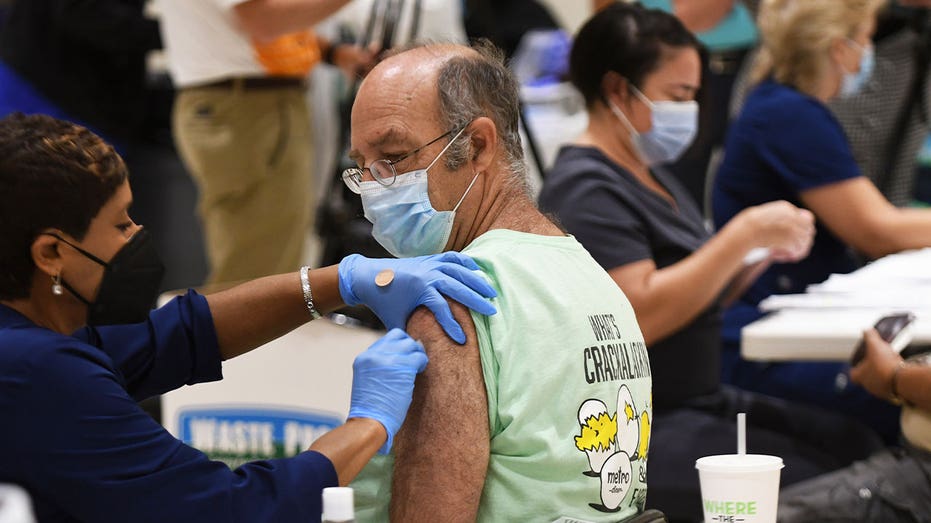
ALTAMONTE SPRINGS, FLORIDA, UNITED STATES - 2021/08/18: A nurse administers a COVID-19 booster shot to Joe Rigdon at a vaccination site in Eastmonte Park, Altamonte Springs. (Paul Hennessy/SOPA Images/LightRocket via Getty Images)
Walensky overruled her advisers, saying that tens of millions of people who work in high-risk places should be added to the list.
Now, the FDA will reportedly weigh whether a Moderna booster should contain half of the original dose, what the best time for a second shot of the single-dose Johson & Johnson vaccine is and examine the potential effectiveness and safety of using multiple brands of vaccines.
CLICK HERE TO READ MORE ON FOX BUSINESS
While all three vaccines still offer strong protection against the virus and death from COVID-19, White House officials announced in August – amidst a delta variant outbreak – that boosters would be offered to nearly all adults.
However, many have argued that there is little information regarding breakthrough infections and whether or not boosters would be necessary for those who are already fully vaccinated.
CDC data shows that more than 103 million Americans are fully vaccinated with Pfizer's vaccine, 69 million with Moderna and 15 million with Johnson & Johnson.
Merck seeking FDA authorization for COVID pill
Merck eyes FDA approval for COVID-19 pill. FOX Business' Jackie DeAngelis with the more.
In total, more than 187.2 million people are fully vaccinated and nearly 7.8 million people have received a booster dose.
Federal regulators meet as vaccinations have increased by more than 50% over the past couple of weeks due to employer vaccine mandates and the Pfizer boosters.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Members of the public who wish to present data, information or views to the FDA committee may do so electronically or by mail.
A docket – under the number FDA-2021-N-0965 – was established and will close on Wednesday. Comments received before Tuesday will be provided to the committee, while those after Tuesday and by Wednesday will be taken into consideration by the FDA.
The Associated Press contributed to this report.






















