FDA commissioner 'very optimistic' about coronavirus plasma treatment
Coronavirus survivors' blood might help others develop an immunity to the virus
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
Food and Drug Administration Commissioner Dr. Stephen Hahn said Tuesday that he is "very optimistic" about the possibility of a coronavirus plasma treatment.
Hahn said such a treatment is promising as a therapy for COVID-19 patients and a potential preventative treatment for others who haven't been infected yet on "Mornings with Maria."
"I'm very optimistic about the convalescent plasma," Hahn said. "Convalescent plasma is where you take plasma -- the liquid, protein part of the blood that contains natural immunity that people have developed when they've been exposed to COVID-19 -- you process it, and you give it to someone who's sick."
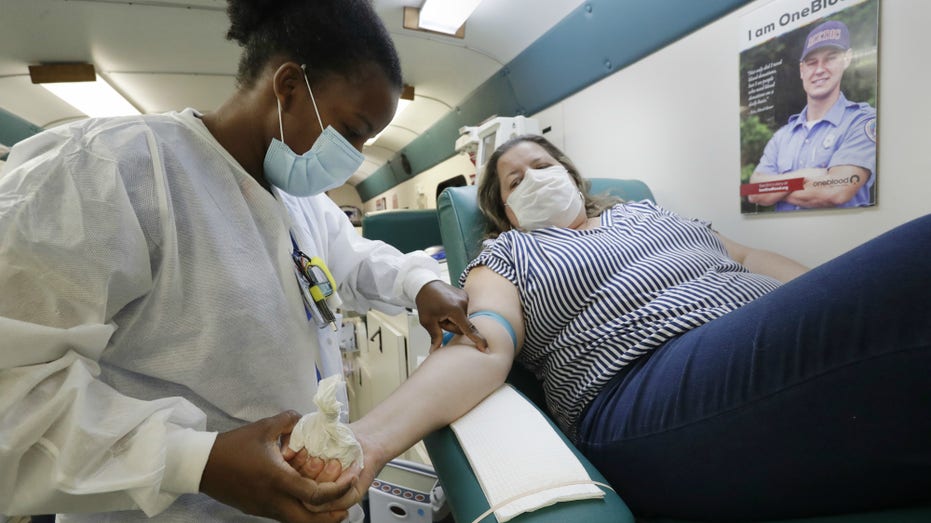
Phlebotomist Josianne Fiefie, left, looks for a vein as Maria Teresa Andreychuk prepares to donate blood aboard a OneBlood blood donation bus April 22, 2020, in Miami. (AP Photo/Wilfredo Lee)
When a body gets infected with a virus like COVID-19, it starts producing antibodies that fight a specific germ. These antibodies can be found in the blood of COVID-19 survivors for months or even years after they catch the virus.
CORONAVIRUS SURVIVORS' BLOOD KEY FOR FIGHTING ILLNESS
The FDA commissioner explained that "there are some good preliminary data to suggest that it is a benefit," and Red Cross and Mayo Clinic developers are scaling up their plasma treatments in anticipation of higher demand for the treatment that could help contain COVID-19.
WHAT IS PLASMA THERAPY FOR CORONAVIRUS TREATMENT AND DOES IT WORK?
Convalescent plasma can also be used to produce "hyperimmune globulin," Hahn said, "which is a shot of concentrated antibodies that can also provide a treatment, and could also potentially be used as a prophylactic for those who haven't been exposed to the virus."
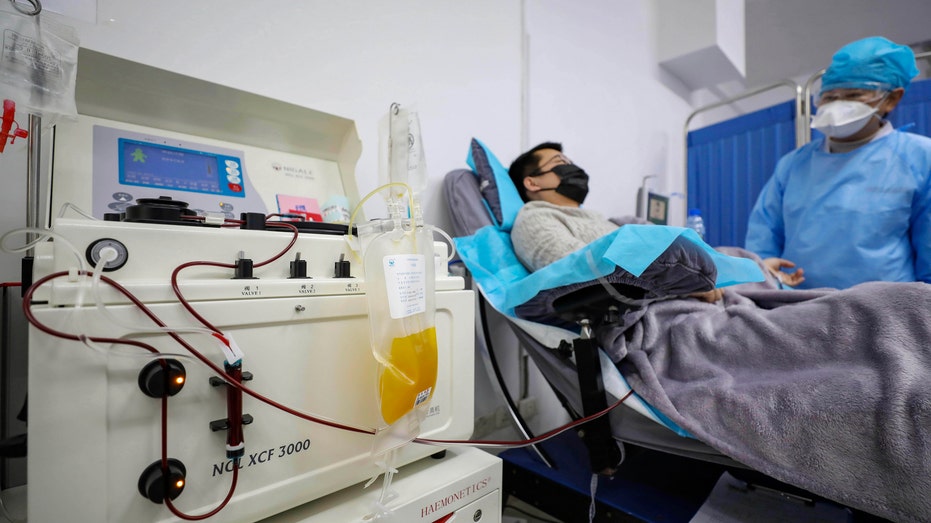
Dr. Kong Yuefeng, a recovered COVID-19 patient who has passed his 14-day quarantine, donates plasma in the city's blood center in Wuhan in central China's Hubei province Feb. 18, 2020. (Chinatopix via AP)
Hahn is also optimistic about COVID-19 testing, saying that the U.S. has ramped up its testing from just "tens of thousands" about two months ago to "5.5 million" this week. He added that there are more than 390 test developers actively working with the FDA to produce and distribute tests.
The FDA is still conducting trials for other potential COVID-19 treatment drugs such as Gilead's remdesivir antiviral drug and an immunosuppressive drug touted by President Trump called hydroxychloroquine.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The administration is looking at whether these drugs help patients already diagnosed with COVID-19 and whether they can prevent medical professionals and first responders from catching the virus when they come in contact with infected people.




















