Moderna launches coronavirus vaccine Phase 3 trial
Moderna anticipates enrolling 30,000 US participants
Massachusetts biotech company Moderna launched Phase 3 of its coronavirus vaccine trial on Monday.
Moderna anticipates enrolling 30,000 U.S. participants.
MODERNA GETS FURTHER $472M US AWARD FOR CORONAVIRUS VACCINE DEVELOPMENT
There's still no guarantee that the experimental vaccine, developed by the National Institutes of Health and Moderna, will really protect the participants.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 49.70 | +3.10 | +6.65% |
The needed proof: Volunteers won’t know if they’re getting the real shot or a dummy version. After two doses, scientists will closely track which group experiences more infections as they go about their daily routines, especially in areas where the virus still is spreading unchecked.
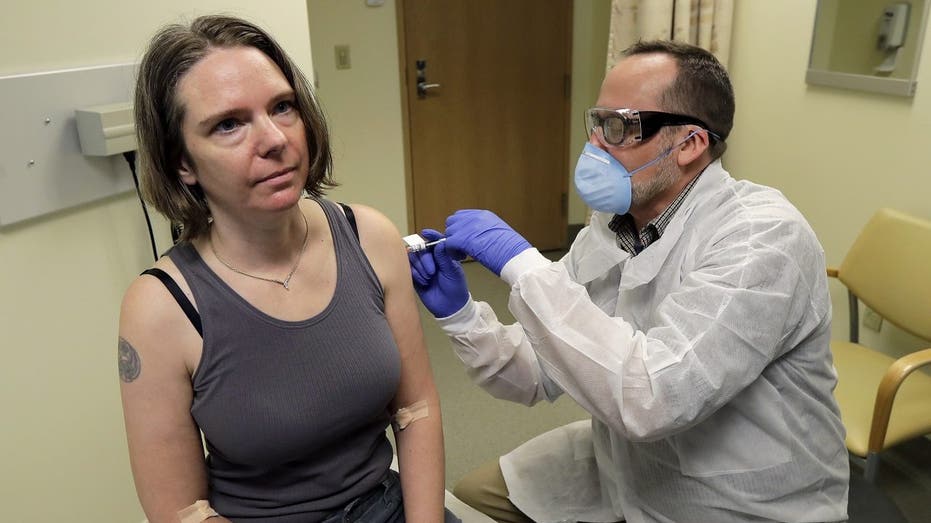
A pharmacist gives Jennifer Haller, left, the first shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19, March 16, 2020, at the Kaiser Permanente Washington Health Research Institute in Seattle. (AP Photo/Ted S. Wa
Moderna is working with the NIH and Biomedical Advanced Research and Development Authority to develop its vaccine as part of Operation Warp Speed, the Trump administration's effort to pinpoint and accelerate the most effective coronavirus vaccines.
The drugmaker announced Sunday it was awarded $472 million more via BARDA to help develop its potential vaccine.
Moderna's stock surged in mid-July following positive results from the company's "Phase 1" study of its mRNA-1273 COVID-19 vaccine candidate.
The Associated Press contributed to this report.
GET FOX BUSINESS ON THE GO BY CLICKING HERE




















