Moderna shares tank after FDA delays COVID vaccine for 12- to 17-year-olds
Four Nordic countries recently decided against recommending vaccine for age group
Expect rising COVID cases in winter: Dr. McCarthy
Infectious disease expert Dr. Matt McCarthy discusses COVID as winter approaches and the FDA approving Pfizer vaccine for children.
Shares in Moderna Inc. dropped at least 5% as trading opened Monday after a delay in the decision to approve the COVID-19 vaccine for adolescents ages 12-17.
The U.S. Food and Drug Administration (FDA) on Friday notified Moderna that the agency would require additional time to review and evaluate possible risks associated with the vaccine – particularly, a possible risk of myocarditis after vaccination.
The FDA delay could last several weeks, but the timing is unclear. The agency merely plans to further review data before making a final decision.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.21 | +0.73 | +2.78% |
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
| JNJ | JOHNSON & JOHNSON | 240.00 | +2.24 | +0.94% |
However, that delay alone was enough to spook some investors into dumping their Moderna shares. The stock dropped 5% after trading started Monday morning. As of 10 a.m., the stock price had gone up and down again, and the value was down by about 6%.
In a press release Sunday, Moderna noted that an increased risk of myocarditis has been noted for COVID-19 vaccines, including the Moderna-produced vaccine. Moderna further noted that the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) have said the risk for this condition has been rare and generally mild.

A sign for the Food And Drug Administration is seen outside of the headquarters on July 20, 2020 in White Oak, Maryland. (Photo by Sarah Silbiger/Getty Images / Getty Images)
FDA APPROVES PFIZER VACCINE FOR EMERGENCY USE IN CHILDREN AGED 5 TO 11
The most notable example of cases occurred in four Nordic countries – Denmark, Finland, Norway and Sweden – that decided to recommend against the use of Moderna’s COVID-19 vaccine for younger age groups, The Wall Street Journal reported.
The European Medicines Agency (EMA) said Thursday that new preliminary data from those four countries supports a warning adopted in July that inflammatory heart conditions can occur in very rare cases. The EMA warning also applied to the Pfizer-BioNTech COVID-19 vaccine.
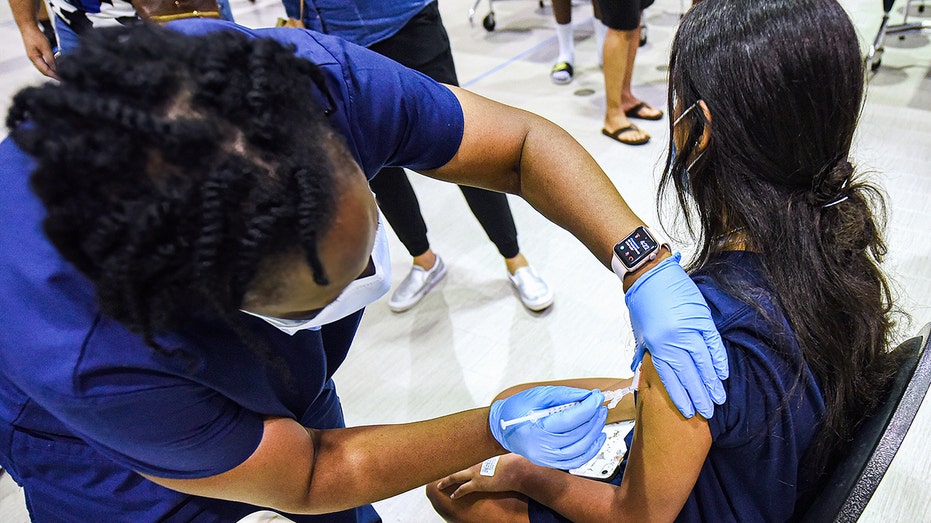
A nurse gives a girl a dose of the Pfizer vaccine at a COVID-19 vaccine clinic at Lyman High School in Longwood, Florida, on the day before classes begin for the 2021-22 school year. (Photo by Paul Hennessy/SOPA Images/LightRocket via Getty Images) (Getty Images)
DOW JONES, S&P, NASDAQ OPEN NOVEMBER AT RECORD HIGHS
A Moderna spokesperson said Thursday that the company was aware of the decision in Europe and noted that COVID-19 itself increases the risk of these heart conditions.

Stephane Bancel, chief executive officer of Moderna Therapeutics Inc., stands for a photograph at the company's office in Cambridge, Mass., on Tuesday, Nov. 14, 2017. (Adam Glanzman/Bloomberg via Getty Images | AP Photo/Gustavo Garello / Getty Images)
Moderna estimated that around 1.5 million adolescents have received the Moderna COVID-19 vaccine and that the observed rate of myocarditis in adolescents under the age of 18 do not suggest an increased risk for the condition.
ARKANSAS WILL ‘LOSE VALUABLE WORKERS’ OVER COVID VACCINE MANDATE: GOV. HUTCHINSON
"Moderna is committed to conducting its own careful review of new external analyses as they become available," the company said in its statement Sunday. "The Company does not yet have access to data from some recent international analyses."
The movement of Moderna stock indicates that some shareholders are not confident that Moderna will receive approval and that the FDA may instead follow the EMA and Nordic examples.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Moderna did however confirm that it will now delay filing a request for Emergency Use Authorization (EUA) for use of its COVID-19 vaccine for an even younger group aged 6 to 11 years old.
The FDA last week authorized the use of the Pfizer COVID-19 vaccine in that age group.





















