US, Pfizer reach $3.2B COVID vaccine deal
Pfizer is seeking FDA approval for its oral antiviral treatment Paxlovid
Pfizer and BioNTech SE said Wednesday that they had inked a $3.2 billion agreement with the U.S. government for 105 million doses of their COVID-19 vaccine.
The deal includes new versions of the shots, and they would be delivered by the fall, pending a decision by the Food and Drug Administration (FDA).
Federal officials say the deal includes the option to purchase a total of 300 million vaccine doses.
This comes as Pfizer said Thursday that it is seeking full approval for its oral COVID antiviral treatment Paxlovid by the FDA.
BIONTECH, PFIZER TO START TESTING UNIVERSAL VACCINE FOR CORONAVIRUSES
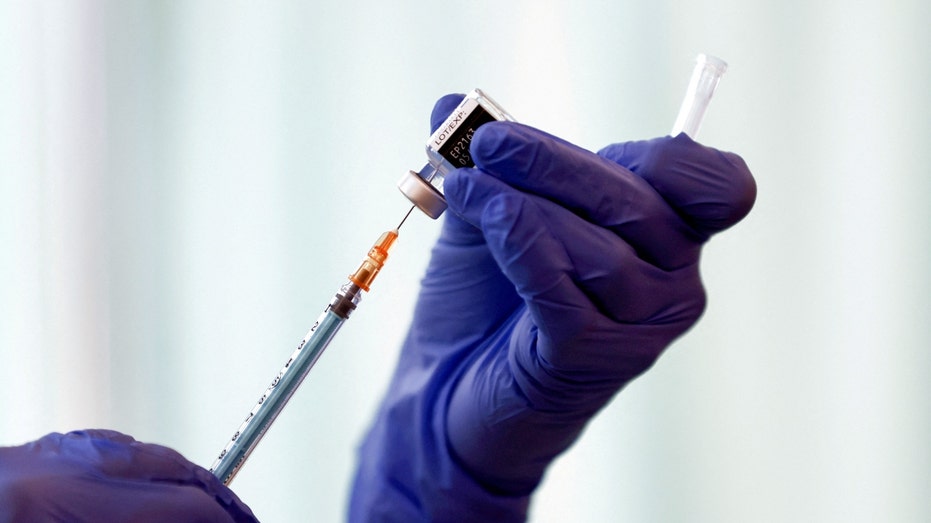
FILE PHOTO: A medical worker fills a syringe with a dose of the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine as Japan launches its inoculation campaign, at Tokyo Medical Center in Tokyo, Japan February 17, 2021. (Behrouz Mehri/Pool via REUTERS/File Photo / Reuters)
The drug would be used for the treatment of COVID-19 in vaccinated and unvaccinated people at high risk for progression to severe illness.
Paxlovid is currently authorized for emergency use for the treatment of mild-to-moderate COVID-19 in adults and kids ages 12 years and older, weighing at least 88 lbs, with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19.
The vaccine-maker said that, according to the Centers for Disease Control and Prevention (CDC), 50-60% of the U.S. population is estimated to have one or more risk factors for progressing to severe COVID-19 illness.
"As the COVID-19 pandemic continues to evolve and be highly unpredictable, we must remain vigilant in protecting those who are at greatest risk of getting very sick from COVID-19, as they remain vulnerable to potential hospitalization or even death," Albert Bourla, Pfizer's chairman and CEO, said in a statement. "Data from our clinical development program, coupled with the more than 1.7 million patients around the world who have been prescribed our oral treatment to date, reinforce Paxlovid as an important treatment option for mild-to-moderate COVID-19 in patients at greater risk of progression to severe symptoms, regardless of vaccination status. We look forward to working with the FDA toward full regulatory approval for Paxlovid."

FILE PHOTO: Test tubes are seen in front of displayed Pfizer and BioNTech logos in this illustration taken, May 21, 2021. (REUTERS/Dado Ruvic/Illustration/File Photo / Reuters)
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Pfizer said the submission was supported by non-clinical and clinical data for Paxlovid, including results from a Phase 2/3 EPIC-HR study (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) which found that treatment with Paxlovid reduced the risk of hospitalization or death from any cause by 88% in non-hospitalized, high-risk adult patients treated within five days of symptom onset, in comparison with placebo.
Results from the final Clinical Study showed an 86% reduction in relative risk.
Pfizer said the data analyses included data from both vaccinated patients with and unvaccinated patients without risk factors for severe COVID-19.

FILE - A syringe is prepared with the Pfizer COVID-19 vaccine at a vaccination clinic at the Keystone First Wellness Center in Chester, Pa., Dec. 15, 2021. (AP Photo/Matt Rourke, File) / AP Newsroom)
"While the novel primary endpoint of self-reported, sustained alleviation of all symptoms for four consecutive days was not met, the data were supportive of the efficacy and safety data observed in EPIC-HR for use in patients at increased risk of progression to severe COVID-19 illness," it said.
CLICK HERE TO READ MORE ON FOX BUSINESS
As of the end of May, Pfizer had shipped more than 12 million treatment courses of Paxlovid to nearly 40 countries.
According to data from the Department of Health and Human Services (HHS), more than 1.6 million courses of Paxlovid have been administered in the U.S.
Reuters and The Associated Press contributed to this report.





















