Bernie Sanders bristles when FDA commissioner says he was not aware of Moderna CEO 'golden parachute'
Bernie Sanders was surprised FDA chief Robert Califf was 'not aware' of the Moderna payment
Bernie Sanders bristles when FDA commissioner says he doesn't keep up with Moderna CEO 'golden parachute'
FDA Commissioner Robert Califf was questioned by Sen. Bernie Sanders, I-Vt., during a Senate committee hearing Thursday, June 16, 2022.
Sen. Bernie Sanders, I-Vt., during a Senate committee hearing on the federal response to the COVID-19 pandemic, brought up a nine-figure "golden parachute" payment that Moderna CEO Stephane Bancel received, criticizing the huge sum that came after the vaccine developer received billions in government funding.
Sanders was not just upset about the payment itself, but about how FDA Commissioner Robert Califf was not aware of it. When asked about the payment, Califf said he was "not aware of that," as it was "not something I would keep up with" in his job.
"Not something you would keep up with?" Sanders asked, questioning why the FDA commisioner would not be interested in a company giving $926 million to an executive when they are supposed to be producing vaccines. "If the federal government gives a company two and a half billion dollars and a short time later the head of a company gets a $900 million golden parachute, that is not a concern to you?"
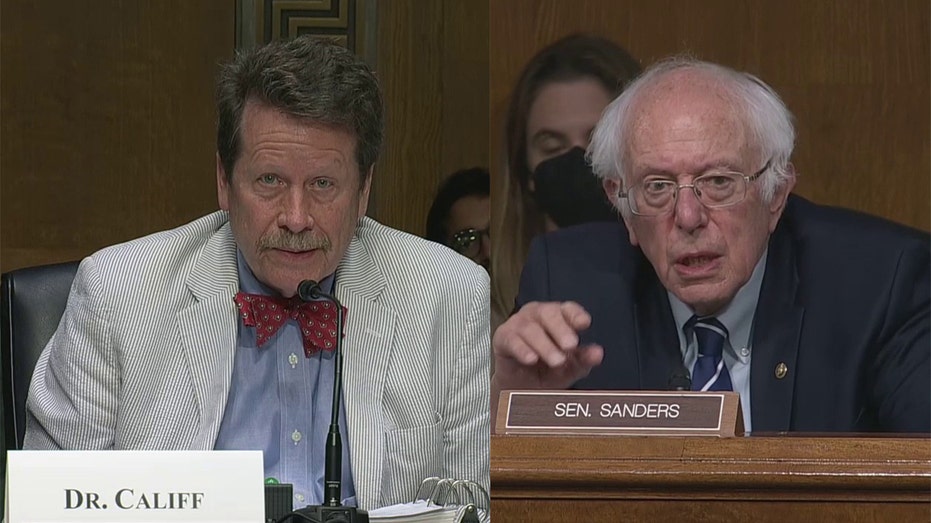
FDA Commissioner Robert Califf was questioned by Sen. Bernie Sanders, I-Vt., during a Senate committee hearing Thursday, June 16, 2022. (Senate Pool Video)
Califf stated he never said it was not a concern for him, just that he does not typically follow such events. What he is concerned with, he said, is "equitable distribution of vaccines that save lives and antivirals that save lives," and how the U.S. is falling short of their goals on that front.
MODERNA'S FIRST-QUARTER PROFIT TRIPLES ON VACCINE SALES
Sanders was not impressed with Califf's answer, stating that he "would hope everybody agrees" that the U.S. needs the appropriate resources in place to get vaccines to everyone who needs them to combat the COVID-19 pandemic.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 41.95 | +0.94 | +2.29% |
"But if one guy ends up with $900 million, rather than using that money to get out the medicine we need, the vaccines we need out to the people, doesn’t make a whole lot of sense to me," he said.
MODERNA LAWSUITS COULD LEAVE TAXPAYERS ON THE HOOK AFTER COMPANY MADE BILLIONS

Robert Califf faced questioning from Sen. Bernie Sanders over a golden parachute payment to the CEO of Moderna (AP Photo/Manuel Balce Ceneta)
The hearing took place a day after an FDA panel recommended vaccines from Moderna and Pfizer to be given to children as young as six months old. Moderna's vaccine for children, meanwhile, is a quarter the dose of their adult version and comes in two shots,
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The FDA is expected to give final approval to the vaccines for children in the coming days. An advisory committee to the CDC is scheduled to meet later this week, clearing the way for the vaccines for kids to hit shelves as soon as next week.
FOX Business' Paul Best and The Associated Press contributed to this report.