AI and machine learning may speed drug development, manufacturing: FDA
The FDA's papers aim to spur conversation about AI and machine learning with patient groups, health care providers and pharmaceutical companies
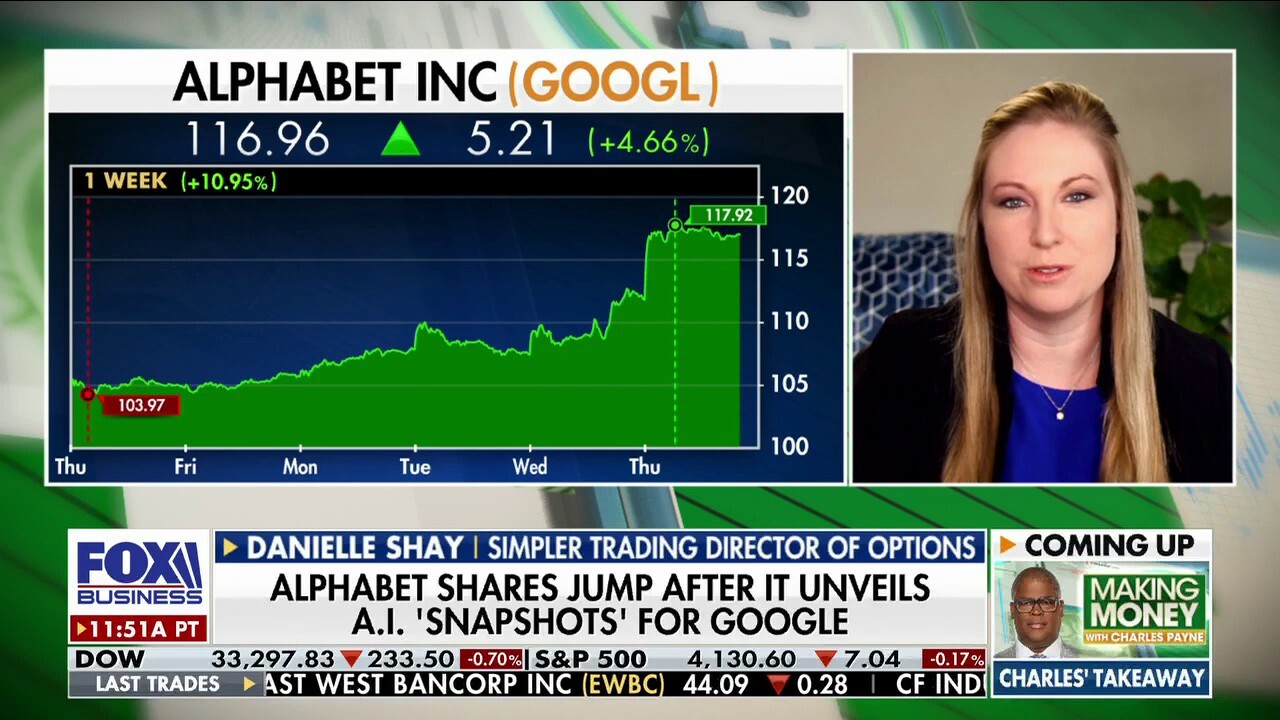
Is Google back in its artificial intelligence game?
Simpler Trading Director of Options Danielle Shay takes a closer look at the impact of AI on technology on Making Money.
The Food and Drug Administration (FDA) published a pair of discussion papers this week in an effort to outline areas artificial intelligence and machine learning may help in manufacturing pharmaceutical drugs along with regulatory issues it presents.
The FDA’s two discussion papers aim to spur a conversation with stakeholders in the medical community such as pharmaceutical companies, physicians, ethicists, patients and patient advocacy groups and regulatory authorities about the use of artificial intelligence (AI) and machine learning (ML) in developing and producing medications.
"AI/ML’s growth in data volume and complexity, combined with cutting-edge computing power and methodological advancements, have the potential to transform how stakeholders develop, manufacture, use and evaluate therapies," Patrizia Cavazzoni, director of the Center for Drug Evaluation and Research, wrote.
Ultimately, AI/ML can help bring safe, effective and high-quality treatments to patients faster."
NEW AI TOOL HELPS DOCTORS STREAMLINE DOCUMENTATION AND FOCUS ON PATIENTS

The Food and Drug Administration released a pair of discussion papers about the applications of AI and machine learning to develop and manufacture new drugs. (Sarah Silbiger/Getty Images / Getty Images)
Cavazzoni noted that AI and machine learning may help health care providers scan medical literature for findings and offer predictions about how individual patients may respond to certain treatment options and whether they may be more at risk of side effects.
She also wrote that AI chatbots may help answer questions about participation in clinical trials or reporting adverse events, or even create digital "twins" of patients to model a medical intervention before a real-life intervention is done with a human patient.

Artificial intelligence and machine learning may have a significant impact on the development and manufacturing of medical products. (iStock / iStock)
The FDA is already seeing an influx of drug and biological product applications referencing the use of AI and machine learning as those technologies have advanced in recent years. More than 100 submissions in 2021 alone mentioned the use of those technologies.
The most common use of AI and ML was in the clinical development and research phase, but they were also deployed in drug discovery, clinical trial enrichment and safety surveillance.
HEAR THIS: GOOGLE USING AI TO BUILD PERSONALIZED HEARING AIDS
The discussion paper by several divisions of the FDA about AI and machine learning in developing drug and biological products touched on several areas where those technologies are being applied or may be applied in the future:
- Early-stage drug discovery, including the use of compound screening and drug design, could be accelerated or made more efficient through the use of AI and ML.
- Nonclinical research can benefit from AI and ML algorithms analyzing complex data sets and models that may improve the accuracy of those applications.
- Clinical trials are identified as "one of the most significant applications" of AI and ML, as they can be leveraged in designing trials, analyzing and interpreting large data sets and improving the operational efficiency of trials.
- Safety surveillance at the post-marketing stage of drug development could leverage AI and ML to process data around adverse events.
- AI could spur the development of novel types of drugs and personalized approaches to treatment.
It also noted there are some risks involved in the use of AI and machine learning in the development of drugs and biological products that may arise due to biases in the data used to train ML algorithms, inaccuracies or incomplete data sets.
Further, the paper emphasized the importance of human-led governance of AI and machine learning to "help ensure adherence to legal and ethical values, where accountability and transparency are essential for the development of trustworthy AI."
FDA DEBATES HEALTH RISKS OF BIRTH CONTROL WITHOUT PRESCRIPTION
Generative AI chatbots could help health care providers and pharmaceutical researchers process information. (Smith Collection/Gado/Getty Images / Getty Images)
The drug manufacturing discussion paper by the FDA’s Center for Drug Evaluation and Research noted areas where AI and machine learning could be applied in pharmaceutical manufacturing processes, such as:
- Using AI models to more quickly identify optimal manufacturing processing parameters or scale-up processes to reduce development time and waste.
- Advanced Process Control, which could use AI to predict the progression of a process in combination with real-time sensor data and improve the understanding of chemical, physical and biological transformations that occur in the drug manufacturing process.
- AI methods can be used to monitor equipment and detect changes that divert from normal performance to trigger maintenance activities and reduce downtime for manufacturing processes. They can also be used to monitor product quality and the quality of packaging that can detect deviations from requirements related to things like packaging, labels and glass vials.
- AI can examine consumer complaints and manufacturing-related deviation reports that contain large volumes of text to identify problem areas and prioritize improvement areas. Similarly, AI can be used to proactively monitor manufacturing operations and set thresholds for corrective and preventive actions.h
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Both FDA papers posed questions to stakeholders regarding the various use cases for AI and machine learning, best practices, barriers to their use, views on transparency, processes for auditing, current or future regulatory frameworks and more.