Moderna CEO on when to expect COVID booster shot to be available
Stéphane Bancel also discussed Q2 earnings, data that shows 93% efficacy 6 months after second dose
Moderna CEO on COVID booster shots
Moderna CEO Stephane Bancel discusses when to expect booster shots.
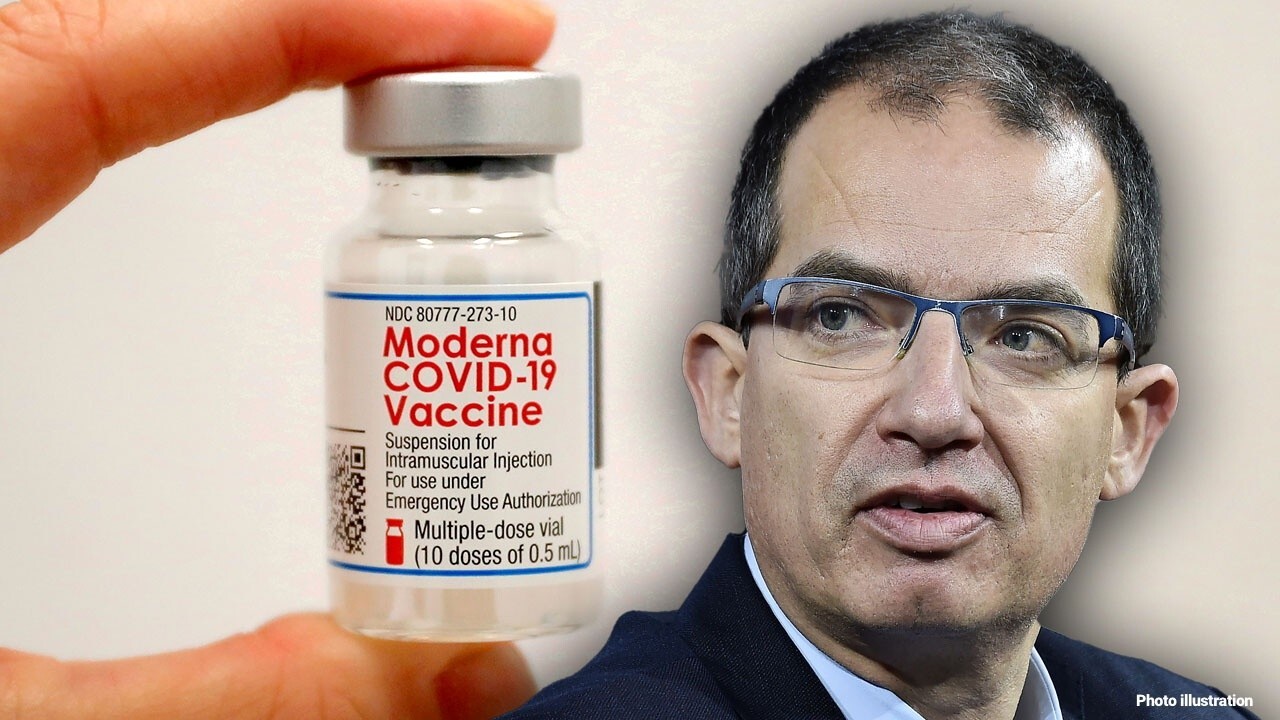
Moderna CEO Stéphane Bancel revealed during an interview with "Mornings with Maria" on Thursday when his company’s COVID-19 booster vaccine could be available to the public as the delta variant spreads across the globe.
He noted that the company is "waiting for a bit more data," but said in some countries, the Moderna booster shot could be available to some demographics "as soon as September."
MODERNA BEING ADDED TO S&P 500
Bancel told host Maria Bartiromo that "we have tried in humans already a booster of the South Africa strain, the Beta virus and we’ve announced this morning that we’re also working on the delta booster and so that data is going to come together in the coming months."
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
He noted that "when we see the totality of the data, we’ll take the best booster."
Bancel then explained that some countries, including Germany, France and Israel have indicated that the elderly population, who received the vaccine in the first wave last December, January and February, could "get the booster as soon as September."
He stressed the urgency of providing the booster shots to that population given the respiratory viruses are "more dangerous in the fall and winter."
"And so it’s important that people who are vaccinated a long time ago, especially with vaccines that have had lower efficacy, be protected so they are not hospitalized this fall and winter," he told Bartiromo.
In May the company announced that two of Moderna’s COVID-19 vaccine booster shots under study induced an immune response against SARS-CoV-2 and variants first identified in South Africa and Brazil.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
A variant-specific booster shot resulted in higher levels of neutralizing antibodies against the concerning South African variant with fewer side effects than a third shot of the original vaccine.
Bancel told Bartiromo on Thursday that the company is "still learning a lot" about the delta variant.
"It’s clearly very contagious," he noted, adding that hospitalizations have been increasing in many places across the country, especially in areas with many unvaccinated people.
He pointed to current data, which revealed that those who received a third dose of a Moderna vaccine had a "42 times increase in antibodies with the same vaccine as a third dose" compared to the level of antibodies provided by a second dose.
"So we think we have the tools to be here to help and to continue to keep Americans safe so that we can run the economy and so kids can go to school," Bancel said.
Moderna CEO on delta variant, booster shots
Moderna CEO Stephane Bancel discusses company earnings, the delta variant threat, vaccine efficacy and what’s ahead.
Bancel provided the updates to his company’s COVID-19 vaccine as the head of the World Health Organization (WHO) calls for a moratorium on administering booster shots in an attempt to help ensure that doses are available in countries where few people have received their first doses.
WHO Director-General Tedros Adhanom Ghebreyesus made the appeal mostly to wealthier countries that have far surpassed the developing world in the numbers of those vaccinated.
In December, Moderna's vaccine was authorized for emergency use in adults in the United States and has since expanded for emergency or conditional use in adults in more than 50 countries, according to Rueters.
Moderna expects to finish its submission for full approval with the U.S. Food and Drug Administration this month.
The company shipped about 100 million doses of their COVID-19 vaccine to the U.S. government in the first quarter of this year and Bancel revealed on Thursday that 200 million doses were shipped in in the second quarter.
"We doubled the output between the first quarter and second quarter," Bancel told Bartiromo, adding that the company is on track to deliver between 800 million and a billion doses this year.
He noted that next year, depending on the dose of the booster, which has not yet been determined given the company is waiting for clinical data, Moderna will likely ship between two to three billion doses.
"We should be able to at least double the output next year, maybe even triple it," Bancel said.
COVID vaccine is 'safe' but shouldn't be mandated: Rep. Crenshaw
Rep. Dan Crenshaw, R-Texas on Veteran Affairs mandating the COVID-19 vaccine for staff and the politicization of the vaccine.
Bancel provided the insight into his company shortly after better-than-expected second quarter earnings were released, revealing the company generated $4.4 billion in revenue and a profit of $2.8 billion, or $6.46 per share, beating quarterly expectations of $5.96 per share.
Analysts polled by Refinitiv were expecting $4.2 billion in sales.
"We had a very strong quarter," Bancel told Bartiromo.
"We are now sitting on $12.2 billion of cash which is why… this morning we announced the board authorized a share buyback of up to $1 billion," he added.
Bancel noted that Moderna has "signed advanced purchase agreements with countries around the world for $20 billion."
"So the business is in great shape and the product is making a lot of progress," he said.
On Thursday, Moderna announced that its COVID-19 shot was 93% effective through six months after the second dose, aligning with the 94% efficacy rate reported in its original clinical trial.
That compares favorably to data released by rival Pfizer-BioNTech last week, which revealed its COVID-19 vaccine’s efficacy drops to around 84% about six months after the second dose.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.21 | +0.73 | +2.78% |
| BNTX | BIONTECH SE | 106.62 | +1.08 | +1.02% |
The study, which has not yet been peer-reviewed and was published on medRxiv, was supported by Pfizer and BioNTech, concluding that despite "a gradually declining trend in vaccine efficacy," it still was "highly efficacious in preventing COVID-19."
Even with Moderna’s strong quarter, the company has not been able to keep pace with the much larger Pfizer, which expects to manufacture as many as 3 billion doses this year and 2021 sales to top $33.5 billion, Reuters reported.
Last month, Moderna joined the S&P 500, the broadest measure of the U.S. stock market, as shares advanced over 280% over the past 12 months.
CLICK HERE TO READ MORE ON FOX BUSINESS
FOX Business' Suzanne O'Halloran, Fox News’ Kayla Rivas and Alexandria Hein, as well as The Associated Press contributed to this report.