CVS, Walgreens administering Pfizer vaccine to kids 12-15 nationwide
CVS is requiring parental or legal guardian consent
CVS and Walgreens are administering COVID-19 vaccines to adolescents between the ages of 12 and 15 as part of the ongoing effort to overcome the pandemic.
Appointments for the Pfizer-BioNTech vaccine at more than 5,600 CVS Pharmacy locations nationwide became available Thursday following Food and Drug Administration (FDA) emergency use authorization for the age group.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| CVS | CVS HEALTH CORP. | 78.35 | +2.02 | +2.65% |
| WBA | NO DATA AVAILABLE | - | - | - |
CLICK HERE TO READ MORE ON FOX BUSINESS
Participating CVS Pharmacy locations will begin administering the shot with parental or legal guardian consent and children must be accompanied by an adult, according to CVS.
Walgreens also opened up appointments Thursday for Pfizer’s COVID-19 vaccine for adolescents ages 12-15. This includes same-day appointments.
The Food and Drug Administration declared that the Pfizer vaccine is safe and offers strong protection for younger teens based on testing of more than 2,000 U.S. volunteers ages 12 to 15. On May 10, the Pfizer-BioNTech vaccine became the first and only FDA-authorized COVID-19 vaccine for adolescents in this age group.
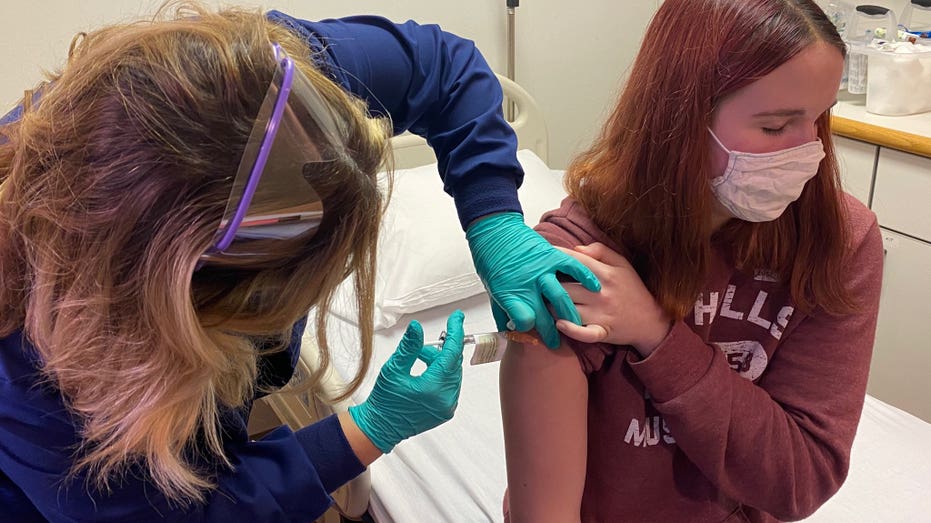
A 16-year-old patient receives a dose of Pfizer's coronavirus vaccine candidate at the Cincinnati Children's Hospital. (Photo courtesy of Cincinnati Children’s)
"Offering vaccinations to younger populations at thousands of locations across the country brings us one step closer to prevailing over the pandemic," CVS Health CEO Karen Lynch said.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
People are encouraged to schedule their appointment online on the CVS or Walgreens websites. Walgreens also suggests that patients use its mobile app or call to schedule an appointment, although walk-ins are welcome.
Authorizing emergency use of the Pfizer shot for the age group marked a "significant step in the fight against the COVID-19," according to acting FDA Commissioner Dr. Janet Woodcock.
CDC PANEL RECOMMENDS PFIZER COVID-19 VACCINE FOR KIDS 12-15
This "allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic," Woodcock said.
Woodcock noted that the agency "undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations," in order to make this decision, which should reassure parents and guardians.
According to the FDA, between March 1, 2020, through April 30, 2021, approximately 1.5 million coronavirus cases in individuals 11 to 17 years of age were reported to the Centers for Disease Control and Prevention (CDC).
The Associated Press contributed to this report.





















