FedEx begins shipping second COVID-19 vaccine, with first Moderna shots expected Monday
The much-needed doses of the coronavirus vaccine are expected to be given starting Monday
FedEx began shipping the second coronavirus vaccine candidate to earn emergency authorization in the U.S. after Moderna, a Massachusetts-based biotech company, received the authorization for its vaccine candidate from an FDA advisory panel on Thursday.
Employees at a factory in the Memphis area and a facility in Olive Branch, Mississippi were boxing up the vaccine developed by Moderna Inc. and the National Institutes of Health.
A truck left the Mississippi facility Sunday morning with a police escort. The much-needed shots are expected to be given starting Monday, just three days after the Food and Drug Administration authorized their emergency rollout.
FDA PANEL ENDORSES MODERNA CORONAVIRUS VACCINE FOR EMERGENCY USE
Later Sunday, an expert committee will debate who should be next in line for early doses of the Moderna vaccine and a similar one from Pfizer Inc. and Germany’s BioNTech. Pfizer's shots were first shipped out a week ago and started being used the next day, kicking off the nation’s biggest vaccination drive.
FedEx said it has been working with McKesson, the company distributing Moderna's vaccine candidate, for months to prepare.
SEE PHOTOS:
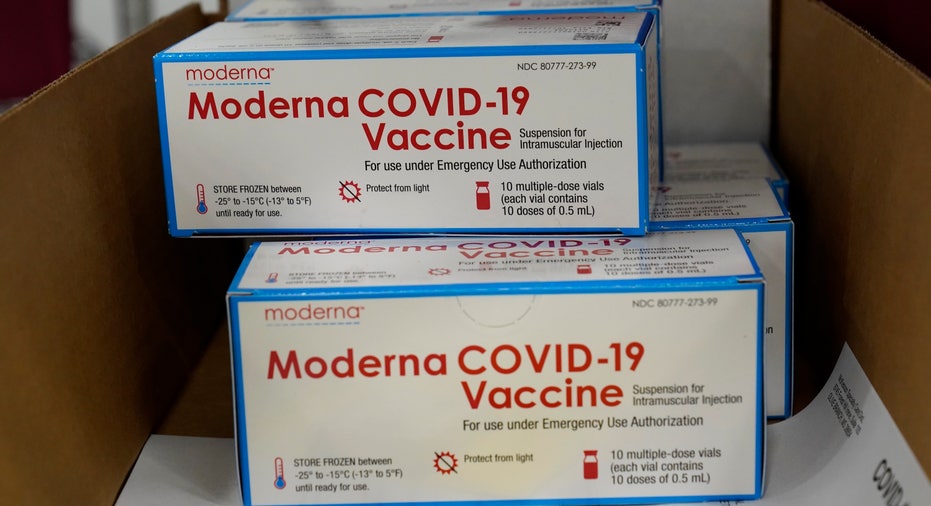
Boxes containing the Moderna COVID-19 vaccine are prepared to be shipped at the McKesson distribution center in Olive Branch, Miss., Sunday, Dec. 20, 2020. (AP Photo/Paul Sancya, Pool)
“The shipment of vaccines to help end the COVID-19 pandemic is among the most important work in the history of FedEx, and our team is focused on the safe and efficient delivery of these critical shipments,” Raj Subramaniam, president and COO of FedEx, said in a statement. “As we have said since the onset of the pandemic and our relief efforts, this is who we are and what we do.”
Moderna agreed to deliver approximately 20 million doses of the vaccine candidate to the U.S. government by the end of December. The Department of Defense, in partnership with the Department of Health and Human Services and CDC in Operation Warp Speed, will distribute the vaccine.
“Healthcare workers have been on the front lines of the fight against the virus and are an inspiration to us all. We look forward to vaccinations of this important population starting this week," Moderna CEO Stephane Bancel said in a statement on Saturday.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
| FDX | FEDEX CORP. | 369.23 | +5.27 | +1.45% |
Both the new Moderna vaccine and the Pfizer-BioNTech shot require two doses several weeks apart. The second dose must be from the same company as the first. Both vaccines appeared safe and strongly protective in large, still unfinished studies.
Public health experts say the shots — and others in the pipeline — are the only way to stop a virus that has been spreading wildly. Nationwide, more than 219,000 people per day on average test positive for the virus, which has killed more than 314,000 in the U.S. and nearly 1.7 million worldwide.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Moderna requested emergency use authorization for its vaccine candidate on Nov. 30. Bancel said his company's coronavirus vaccine has demonstrated a "gamechanger" safety record in ongoing studies, along with 94.5% effectiveness in preventing COVID-19.
"People who did get our vaccine did not get any severe disease, which is of course a gamechanger," Bancel told "Mornings with Maria" in November.
CLICK HERE TO READ MORE ON FOX BUSINESS
The Associated Press contributed to this report.