Johnson & Johnson to begin early trial of COVID-19 vaccine candidate earlier than planned
The trial will evaluate patients' response to vaccination
Johnson & Johnson said it is starting its Phase 1/2a trial of its Covid-19 vaccine in the second half of July, earlier than initially planned.
The trial will evaluate patients' response to vaccination and immune response. It will involve 1,045 healthy adults aged 18 to 55, as well as adults aged 65 and above, J&J said.
CORONAVIRUS VACCINE CANDIDATES' PIVOTAL US TESTING TO START THIS SUMMER
The study will be held in the U.S. and Belgium, the company said.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| JNJ | JOHNSON & JOHNSON | 246.88 | +1.95 | +0.80% |
J&J said it is in discussions with the National Institutes of Allergy and Infectious Diseases to start the vaccine's Phase 3 trial ahead of schedule, depending on the outcome of the Phase 1 studies and regulators' approval.
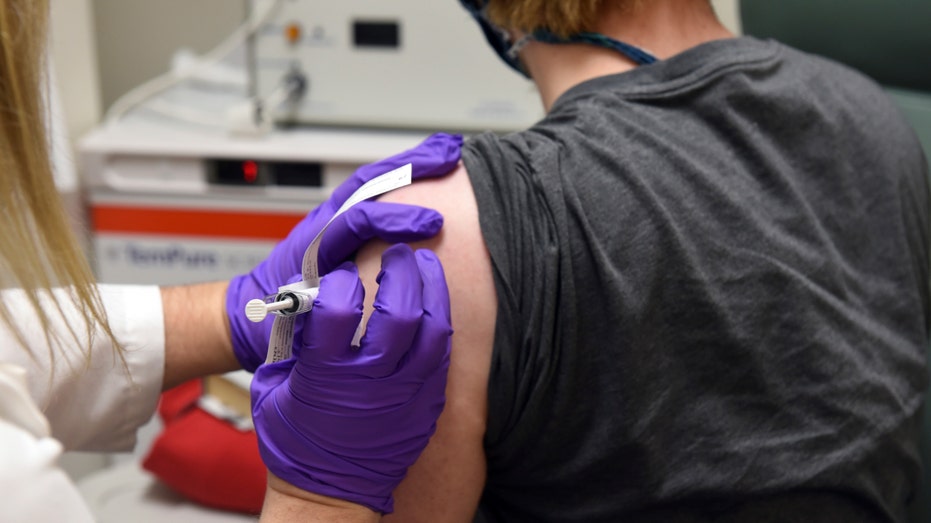
This May 4, 2020 photo from the University of Maryland School of Medicine, the first patient enrolled in Pfizer's COVID-19 coronavirus vaccine clinical trial at the University of Maryland School of Medicine in Baltimore, receives an injection. (Unive
The company said it would supply more than one billion vaccine doses in 2021 if the vaccine is proven to be safe and effective.
CLICK HERE TO READ MORE ON FOX BUSINESS




















