State AGs letter urging availability of coronavirus drug shot down by HHS
Coronavirus drug remdesivir is helping hospitalized patients
The Department of Health and Human Services pushed back against a letter signed by more than 30 state attorneys general concerned that the federal government is not doing enough to get Gilead Sciences' coronavirus treatment remdesivir to the American people.
The letter's request for the government to use the Bayh-Dole Act to grant march-in rights to states to allow other firms to manufacture remdesivir is a non-starter, an HHS spokesperson told FOX Business. The spokesperson said march-in rights only apply to products with intellectual property that was funded by the federal government, meaning all patents underlying the product were conceived or reduced to practice with federal funds.
COVID-19 DRUG REMDESIVIR TO COST $3,120 FOR TYPICAL PATIENT ON PRIVATE INSURANCE
Louisiana Attorney General Jeff Landry and California Attorney General Xavier Becerra, a Republican and a Democrat, led the charge Tuesday to "urge the federal government to exercise its rights under the Bayh-Dole Act, which will allow the National Institutes of Health (NIH) and the FDA to ensure that Americans can afford and access a sufficient supply of remdesivir during this pandemic."
"[W]e think it is clear that Gilead has not established a reasonable price, nor has it met the health and safety needs of the public given the COVID-19 pandemic," the attorneys general wrote. "We urge the federal government to use its march-in rights to help increase the supply of this drug and lower the price so it is accessible to our state residents."
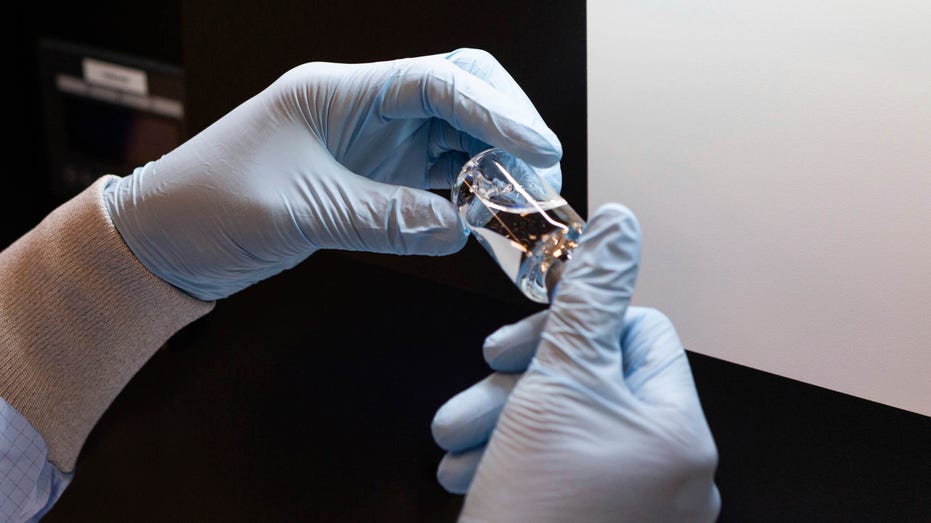
In this March 2020 photo provided by Gilead Sciences, a vial of the investigational drug remdesivir is visually inspected at a Gilead manufacturing site in the United States. (Gilead Sciences via AP)
Such a move would be unprecedented.
"March-in rights described under Bayh-Doyle allow the government, in certain limited circumstances, to force the party with title to a government-funded intellectual property to grant a license to another entity," the National Institute of Standards and Technology says on its website. "To date, the government has never exercised these rights."
In a statement, Gilead said the letter contained "multiple factual inaccuracies." Gilead argues that enabling generic manufacturers to make remdesivir won't help for at least six months. The company will invest more than $1 billion to manufacture more than two million courses of remdesivir in 2020, which it says will exceed projected patient demand.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| GILD | GILEAD SCIENCES INC. | 152.50 | +3.13 | +2.10% |
"The AGs’ letter ... erroneously asserts that remdesivir will increase patient out-of-pocket costs, when the reality is patients will not be billed separately for remdesivir when treated in an inpatient hospital setting," Gilead said in a statement. "The AGs’ letter ignores the significant savings hospitals will see from shortening COVID-19 patients’ average time to recovery by four days, resulting in earlier discharge for many patients."
CLICK HERE TO READ MORE ON FOX BUSINESS
Gilead will charge U.S. hospitals $3,120 for the average patient with commercial insurance.
The full text of Landry and Becerra's letter can be read here.
FOX Business' inquiries to Landry and Becerra were not immediately returned.




















