A future without Alzheimer's: Fundraising walk fights disease that may reach one in four Americans
A throng of thousands staged a 2-mile walk through Lower Manhattan on Saturday to help fund new treatments for Alzheimer's disease, an initiative that progressed this week with drugmaker Biogen's decision to try again for regulatory approval of a new medication.
The 2,463 participants in the ‘Walk to End Alzheimer's,’ had raised $612,234, or 80 percent of their overall goal of $770,000 as of mid-afternoon Saturday. Over 5 million Americans are living with Alzheimer's disease, making it the sixth-leading cause of death in the U.S. and the only disease among the top 10 causes of death that cannot be cured, prevented or even slowed.
The disease and other forms of dementia may affect one in four Americans by 2060, and the cost of care and treatment in 2017 alone was estimated to be $259 billion, according to the Centers for Disease Control and Prevention.
“I watched my mom suffer with Alzheimer’s before she passed a year ago,” Daniel Carpey, 59, told FOX Business at Saturday’s fundraising event. This "brings back a lot of tough memories," he added, "but it’s just nice to be surrounded by people who are also fighting to solve the problem of Alzheimer’s.”
WHY BIOGEN'S ALZHEIMER'S DRUG FAILURE MAY HAVE A SILVER LINING
Others expressed optimism over Biogen’s second attempt at winning Food and Drug Administration approval for its new treatment, which showed promising results after clinical trials at 350 sites in 20 countries.
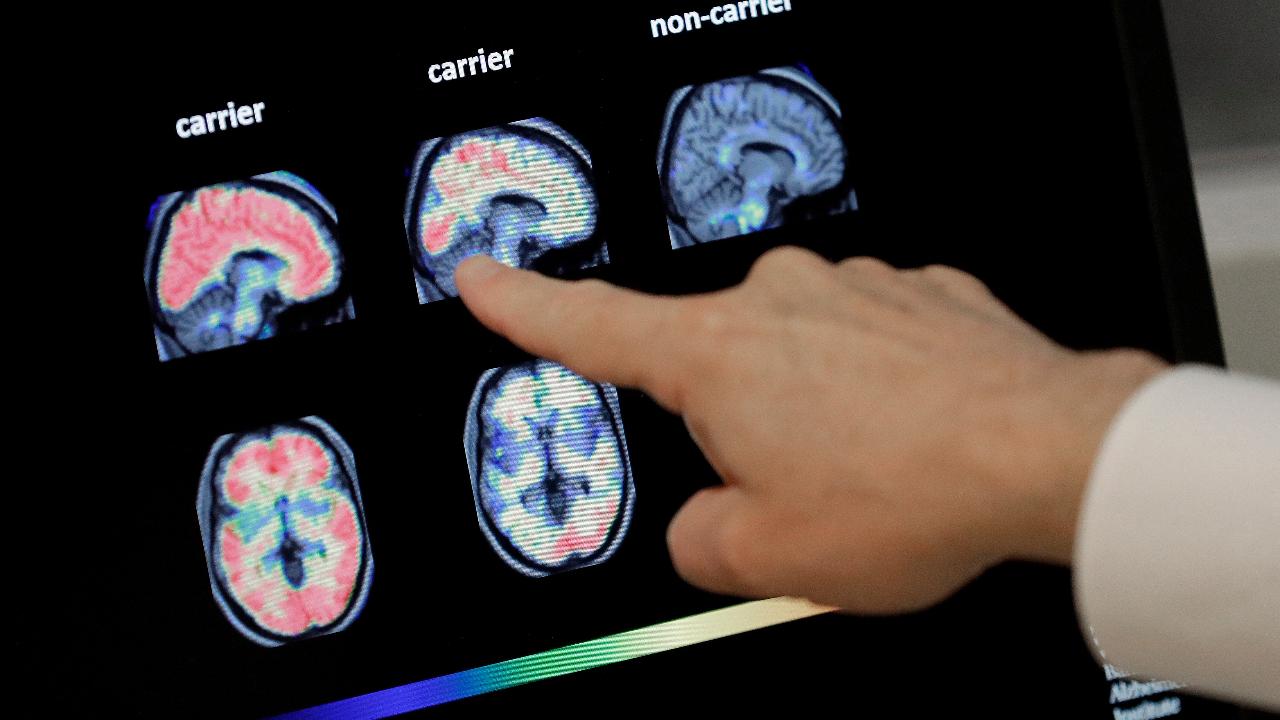
(Matthew McNulty)
More than 3,200 Alzheimer’s patients participated in the studies, and a report released Tuesday showed users displayed as much as 27 percent less decline in cognitive abilities after 18 months of aducanumab infusions than patients not receiving them.
While the $50 billion biotech company’s first attempt to win FDA approval for aducanumab failed in March, after a fuller review of a larger set of data, the drug was found to help people with early stages of the disease, according to Biogen’s most recent study.
THIS COMPANY SAYS IT WILL SEEK APPROVAL OF ALZHEIMER'S DRUG
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| BIIB | BIOGEN INC. | 201.18 | +15.82 | +8.53% |
Based on recent discussions with the FDA, the Massachusetts-based company plans to seek approval in early 2020.
“With such a devastating disease that affects tens of millions worldwide, today’s announcement is truly heartening,” Biogen CEO Michel Vounatsos said. “We are hopeful about the prospect of offering patients the first therapy to reduce the clinical decline of Alzheimer’s.”
NEW BLOOD TEST CAN IDENTIFY ALZHEIMER'S DISEASE YEARS BEFORE SYMPTOMS
The agency’s goal for approving a drug like aducanumab is six months, meaning after final approval, aducanumab could become available for physicians to prescribe sometime before 2021, according to the FDA's website.
"I lost my father and grandfather to this disease many years ago," said Emmy and Tony Award-winning actor David Hyde Pierce, who joined the Saturday event organized by the New York City chapter of the Alzheimer's Association and has participated many times before. "By being here together, we are honoring our families and fighting for a future without Alzheimer's."