AstraZeneca to mass produce potential coronavirus vaccine
Only a handful of the vaccines in development have advanced to human trials
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
(Reuters) - Britain's AstraZeneca joined forces with the University of Oxford on Thursday to help develop, produce and distribute a potential COVID-19 vaccine, as drugmakers around the world race to find a solution to the deadly disease.
UK Business Secretary Alok Sharma welcomed the tie-up as a vital step to making the Oxford vaccine available as soon as possible if it succeeds in clinical trials.
GILEAD SEES 'POSITIVE' DATA ON CORONAVIRUS TREATMENT REMDESIVIR TRIAL
A team of British scientists last week dosed the first volunteers, and earlier this month said large-scale production capacity was being put in place to make millions of doses even before trials show whether it is effective.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| AZN | ASTRAZENECA PLC | 193.10 | +5.91 | +3.16% |
Only a handful of the vaccines in development have advanced to human trials, an indicator of safety and efficacy - and the stage where most vaccines fail.
“Our hope is that, by joining forces, we can accelerate the globalisation of a vaccine to combat the virus and protect people from the deadliest pandemic in a generation,” AstraZeneca Chief Executive Pascal Soriot said.
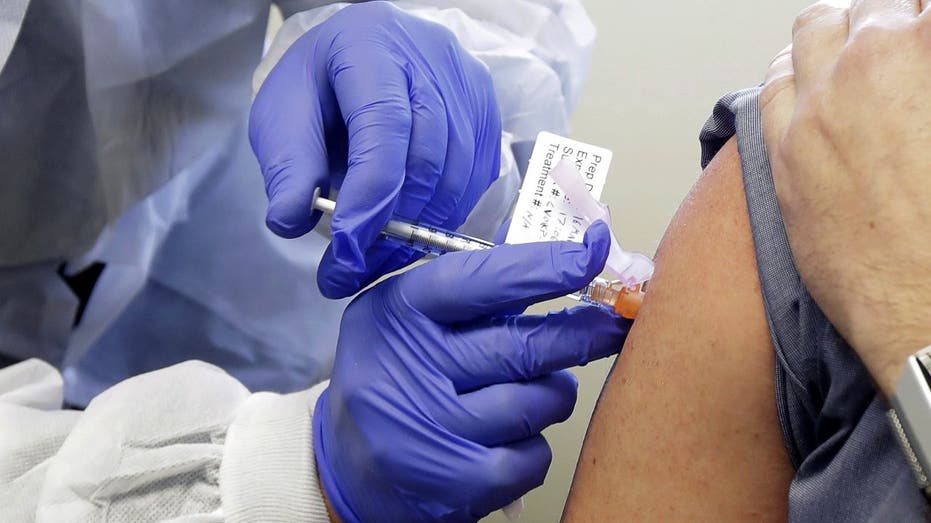
Neal Browning receives a shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19 March 16, 2020, at the Kaiser Permanente Washington Health Research Institute in Seattle. (AP Photo/Ted S. Warren)
The drugmaker did not give details on when it plans to start producing the vaccine “ChAdOx1 nCoV-19”, being developed by the Jenner Institute and Oxford Vaccine Group.
Though the firm is not a major player in vaccine development unlike European peers GSK and Sanofi, who are working on their own vaccine, it has deep pockets and a $6-billion-strong R&D budget.
The AstraZeneca-Oxford partnership is looking to produce 100 million doses by the end of the year and prioritise supply in the UK, Soriot told here the Financial Times.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| AZN | ASTRAZENECA PLC | 193.10 | +5.91 | +3.16% |
Cambridge-based AstraZeneca is also testing two of its approved treatments as a therapy to help in the outbreak that has so far infected over 3 million people and killed more than 215,000.
Its shares rose 2% on London’s FTSE 100 by 0923 GMT as the main index fell, outpacing rival GSK.
Governments, drugmakers and researchers are working on around 100 vaccines for the virus. Industry experts say a successful vaccine will likely take more than a year to be developed but that is much faster than the average development time of 5-7 years.
CLICK HERE TO READ MORE ON FOX BUSINESS
There are currently no treatments or vaccines approved for the highly-contagious respiratory illness caused by the coronavirus, but healthcare workers have been trying many approaches to treat patients.
India's Serum Institute, the world's largest maker of vaccines by volume, has already said here it would produce millions of doses of the Oxford University shot.
The vaccine, a type known as a recombinant viral vector vaccine, uses a weakened version of the common-cold virus spiked with proteins from the novel coronavirus to generate a response from the body’s immune system.
Other drugmakers testing possible COVID-19 vaccines include Pfizer, Moderna, Johnson & Johnson and Novavax.
GET FOX BUSINESS ON THE GO BY CLICKING HERE