Coronavirus vaccine front-runners based in China, Massachusetts
CanSino Biotechnology and Moderna Therapeutics vaccines show promise
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
The race for a coronavirus vaccine has narrowed to two leaders for the time being: China-based CanSino Biotechnology Inc. and Massachusetts-based Moderna Therapeutics.
Moderna was the first U.S. company to get a coronavirus vaccine ready for human testing, and its CEO said the company would put the vaccine at the same price point as similar existing vaccines.
CORONAVIRUS VACCINE DEVELOPMENT COULD COST $1 BILLION
CanSino is pursuing human testing, while the National Institutes of Health began a Phase 1 clinical trial at Kaiser Permanente Washington Health Research Institute in Seattle in mid-March.
CLICK HERE FOR THE LATEST ON CORONAVIRUS
CanSino is known for creating the first Ebola vaccine to gain approval anywhere in the world.
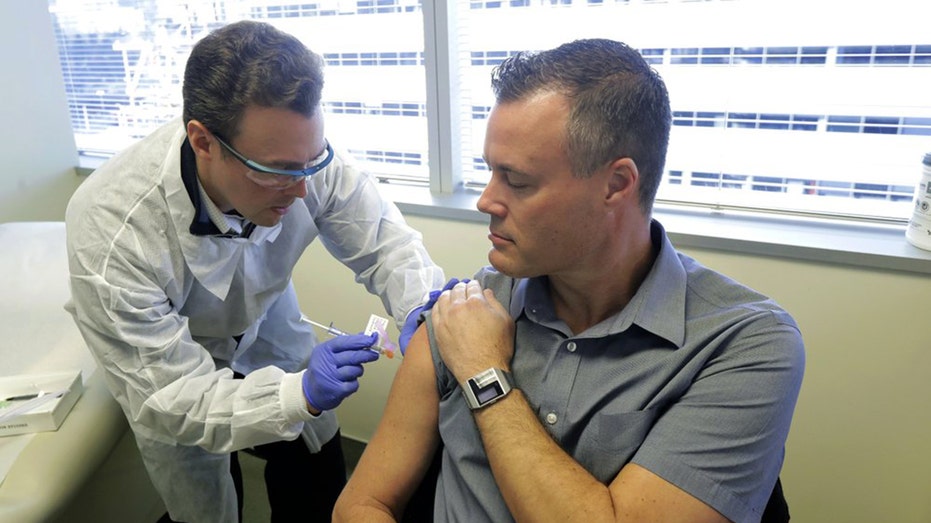
Pharmacist Michael Witte, left, gives Neal Browning, right, a shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19 Monday, March 16, 2020, at the Kaiser Permanente Washington Health Research Institute in Seattle.
Developing such a vaccine could cost well over a billion dollars and take years, vaccine policy expert Kelly Cappio of Avalere Health told FOX Business.
"Vaccine development in general involves ... a high risk of failure. These vaccines can't be developed overnight," Cappio said. "Obviously in an outbreak like this, the timeline is compressed because of an urgent public health need."
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
Both Moderna's and CanSino's share prices are trending up and have gotten boosts in March (CanSino trades on the Hong Kong stock exchange).
Other companies are still in the race for preventing — or curing — the novel coronavirus.
American biotech company Inovio Pharmaceuticals says it created a coronavirus vaccine three hours after getting access to the virus' genetic sequence in mid-January.
"We were able to rapidly construct our vaccine in a matter of about three hours once we had the DNA sequence from the virus available because of the power of our DNA medicine platform," Dr. J. Joseph Kim, Inovio's president and CEO, told FOX Business in February.
A possible treatment that's garnered attention is remdesevir, developed by Gilead Sciences. It's described as an “investigational broad-spectrum antiviral treatment" and has been used to treat patients with Ebola.




















